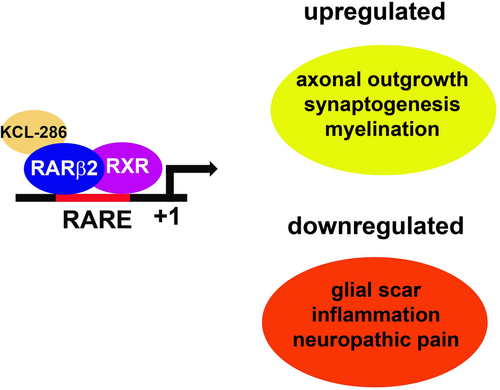
New research from the Institute of Psychiatry, Psychology & Neuroscience (IoPPN) at King’s College London has demonstrated the safety and tolerability of a new drug treatment designed as a therapeutic intervention for spinal cord injury (SCI).
The research, published in British Journal of Clinical Pharmacology, found that the KCL-286 drug – which works by activating retinoic acid receptor beta (RARb) in the spine to promote recovery – was well tolerated by participants in a Phase 1 clinical trial, with no severe side effects. Researchers are now seeking funding for a Phase 2a trial studying the safety and tolerability of the drug in those with SCI.
Published paper: https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.15854
