Volunteers needed to participate in the eWalk trial of non-invasive neurostimulation
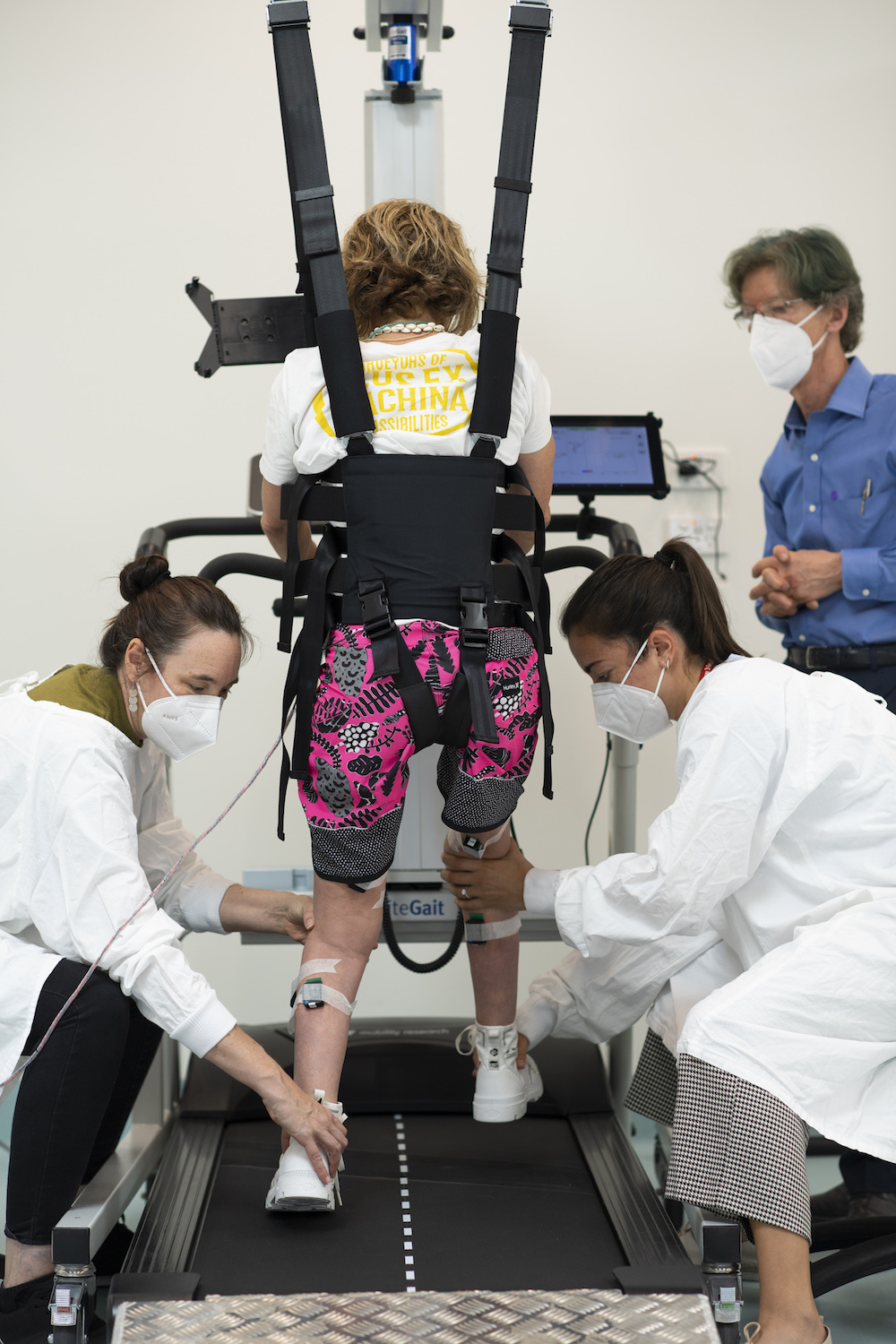
Researchers at NeuRA are seeking volunteer research participants to study the effects of 12 weeks of electrical spinal cord stimulation applied over the skin combined with walking training in paraplegics with incomplete spinal cord injury.
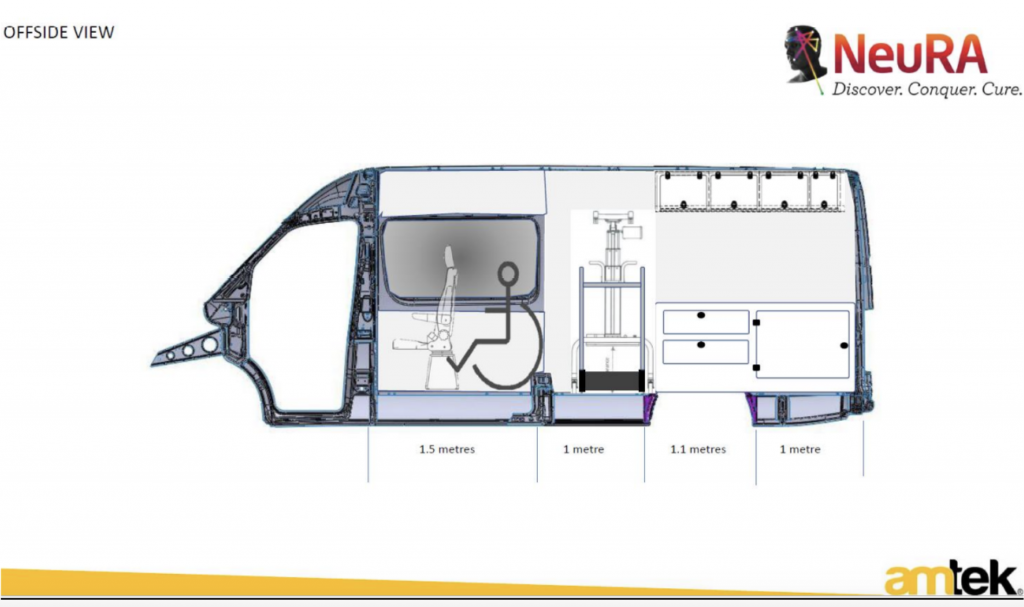
The mobile research lab
The trial requires volunteers to commit to an hours laboratory training three times a week for 12 weeks. Requiring these disabled research participants to travel to NeuRA in Sydney’s eastern suburbs, is not only an enormous burden to the volunteers but would be very expensive in terms of wheelchair taxi fees.
The beauty of this intervention is that it can be delivered to the participant—minimising the personal and cost burdens. NeuRA has designed a Mobile Therapy Gym to outfit a Mercedes-Benz VS30 Sprinter van. This van will be able to house all the equipment required to deliver a session of the transcutaneous spinal stimulation in conjunction with the locomotor training. Further, it will be available to support high-quality SCI research for years to come.
There is an exciting opportunity for a progressive company to sponsor this vehicle.
Please download this information document for more detail.
To find out more contact us on 02 9356 8321 or [email protected]