Volunteers needed to participate in the eWalk neurostimulation trial
Researchers at NeuRA are seeking volunteer research participants to study the effects of 12 weeks of electrical spinal cord stimulation applied over the skin combined with walking training in paraplegics with incomplete spinal cord injury.
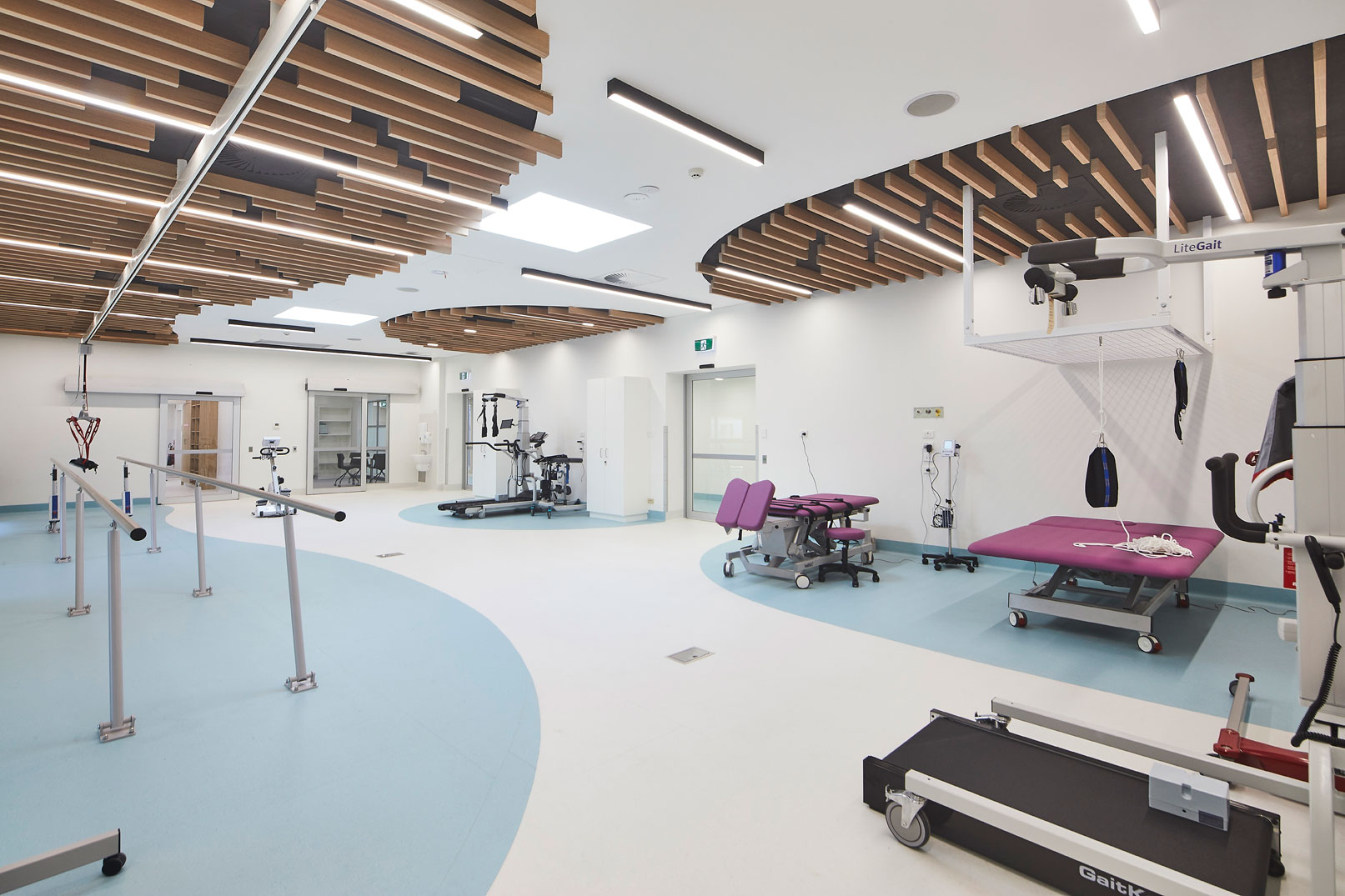
Nine months later, NeuRA’s new Spinal Cord Injury Research Centre (SCIRC) was completed and the first test stimulation experiments commenced — an incredibly cathartic moment for those of us who have been working so long to bring the promise of this line of research to paralysed Australians.
The SCIRC contains an extensive exercise facility complete with gravity assisted walking track and treadmills along with other state-of-the art research equipment. This is surrounded by research labs, offices and wheelchair accessible loos big enough to host a party in.
The research is being led by Professor Simon Gandevia FAA FAHMS FRACP and by Professor Jane Butler PhD, with Dr Bonne Lee MBBS, FAFRAM, and Dr Claire Boswell-Ruys PhD making up the all-star core team.
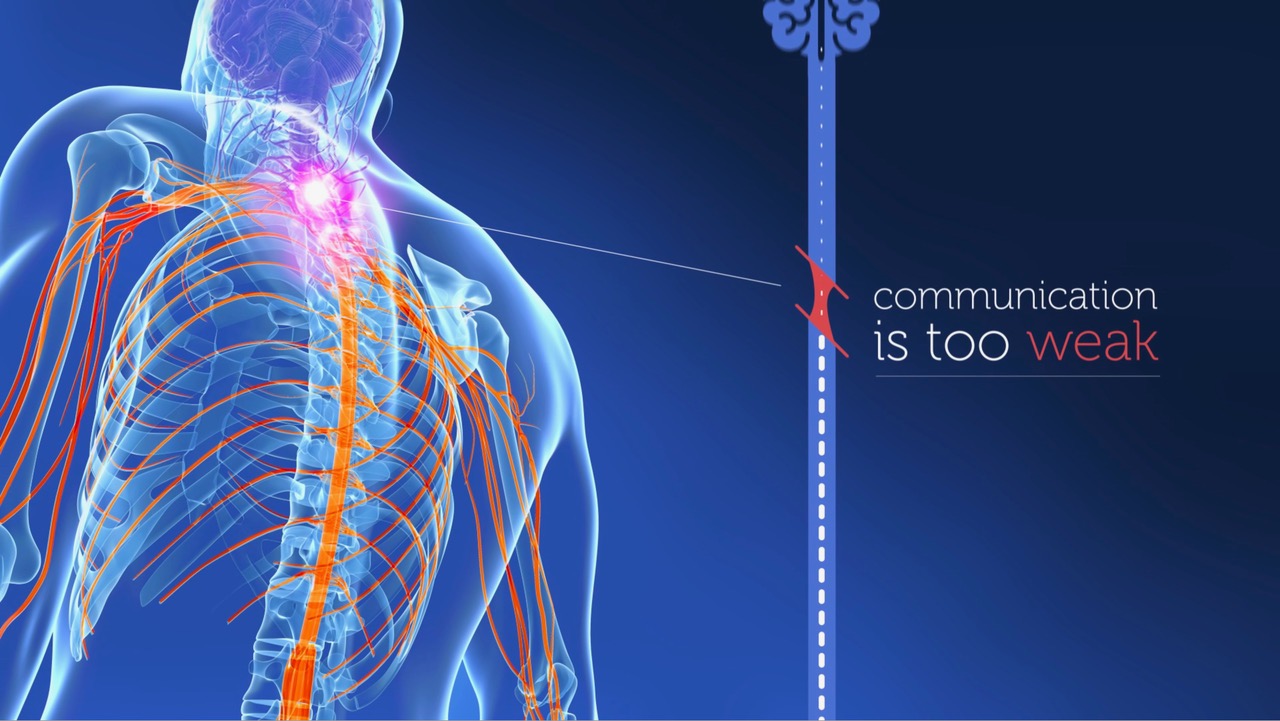
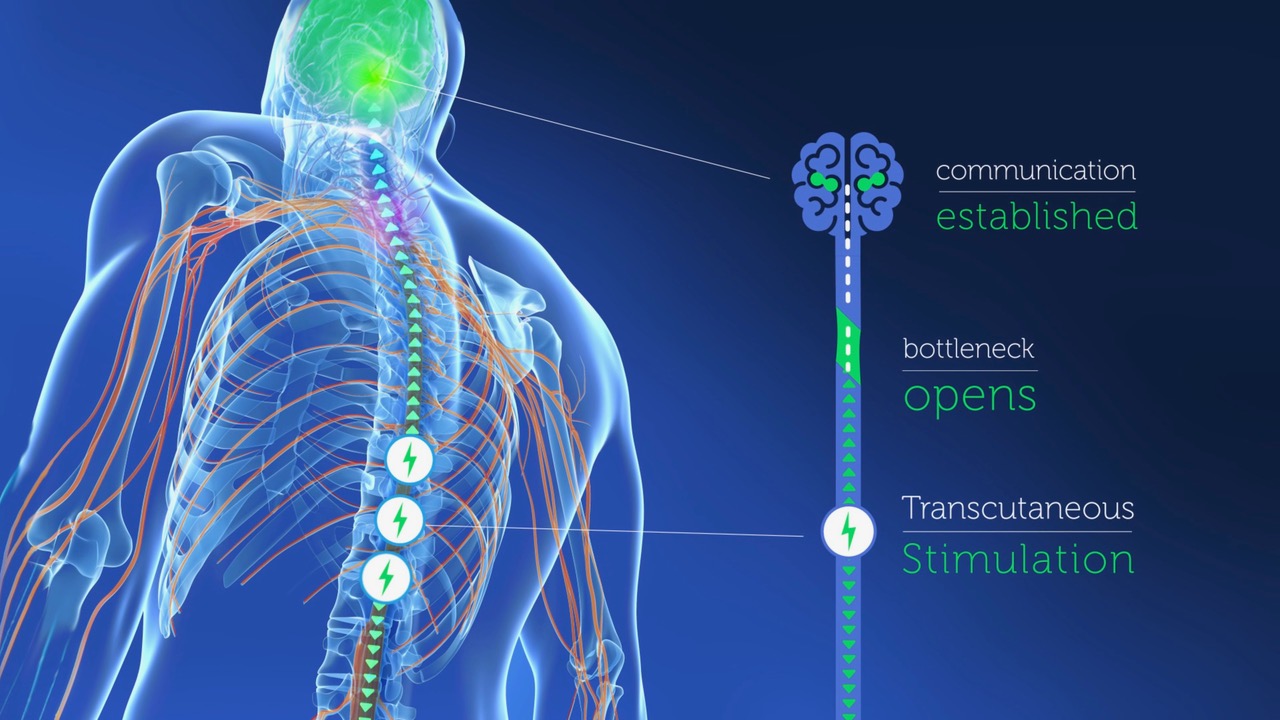
Most of the headline results have used implanted epidural stimulators (see above, “What is neurostimulation”). However, the work at NeuRA is focussing on the ability of “transcutaneous” neurostimulation, which uses electrodes placed on the skin, to restore movement and autonomic function, such as bladder/bowel control, sexual function and cardiovascular stability. This method of applying the stimulation eliminates the high cost and risks of an operation and the long post-operative recovery. Thus, any successful treatment developed could be distributed much more quickly and more widely.
Despite these positive results, a lack of highly controlled clinical trials has prevented this technology from being converted into clinical practice. SpinalCure and NeuRA have partnered to address this need, leading to the SCIRC's flagship clinical trial, the eWalk trial, which commenced treating volunteers in March 2021. The trial is primarily looking at restoring the ability to stand and take steps.
Project Spark will greatly expand on this beginning with a series neurostimulation experiments targeting the returns of function that those affected want most.
Project Spark aims to expand this research around Australia